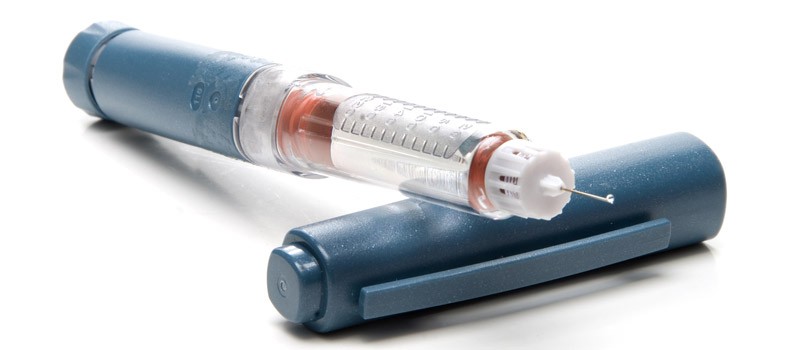
This week, the U.S Food and Drug Administration (FDA) has approved what is being called an “artificial pancreas” for patients with type 1 diabetes.
This automated insulin delivery system, Medtronic’s MiniMed 670G, is a hybrid closed-loop system. It monitors blood sugar and delivers insulin, and shuts off when blood sugar levels are too low. This helps patients with diabetes by removing risks of miscalculating how much insulin to give through injections or with a pump. The device accomplishes insulin deliveries with a small catheter that is inserted under the skin.
While this is a great step toward freedom for patients with type 1 diabetes, it is not a total artificial pancreas. Patients will still need to monitor the amount of carbohydrates in their food and enter the information into the device.
The FDA has approved this device for patients aged 14 and older, and should be available by early 2017. It is being considered a step forward for patients until there is a permanent cure for type 1 diabetes. While similar devices are being developed, this was the first to get approval from the FDA.
“Artificial Pancreas” Approved for Use
Posted On: 09-30-2016
See Related Articles
Blood Sugar’s Ties to Protein and Probiotics Studied
The results of two studies examining the link between protei ...
Posted On: 11-25-2016
read more
New Recommendations for DIY Cloth Masks & What You Can Use
The Centers of Disease Control and Prevention (CDC) recently ...
Posted On: 04-10-2020
read more
Related Doctors
View All-
No record found.
Surgical Procedures
View All-
No record found.
Be Healthy
View All-
No record found.
Health News
Watch & learn
View All
GET A WEEKLY DOSE OF HEALTHY INSPIRATION
Please enter required fields.
Subscription Successful.
Already Subscribed.
Enter Valid Email Address.
Feedback